featured on





The PRoblem
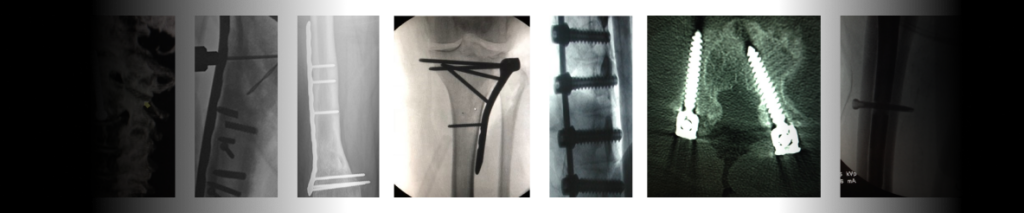
<span data-metadata=""><span data-buffer="">Outdated Tools cause Over-drilling & Improperly installed Screws
The outcomes are as scary as they look. There are many different complications that can arise when correcting distal radius fractures. The most frequent complication is a malunion or misalignment. The effects on patients can range from pain and long-term discomfort to replacement surgery.
53%
of surgeons self-reported errors in the last 6 months
1 in 5
of surgeons self-reported errors in the last 6 months
53%
of surgeons self-reported errors in the last 6 months
our solutions
The Future of Orthopedics
The ONLY drill designed with patient safety in mind. Unprecedented safety features like edge-detection and auto-stop help to keep patients safe and prevent complications.
Infinitely adjustable to improve alignment and patient outcomes.

Our vertical integration allows us to spin up custom solutions – fast.
“
Testimonial
<span data-metadata=""><span data-buffer="">Redefining the Technique of Depth Sensing

“The IntelliSense® Drill represents a quantum leap in drill and depth measurement. The IntelliSense® Drill redefines the technique of depth sensing with unparalleled accuracy that will lessen radiation, minimize screw wastage, and improve patient safety.”
Scott Kozin, MD | Shriners Hospitals for Children – Philadelphia

our solutions
<span data-metadata=""><span data-buffer="">Invest in 100% In-House Engineering & Manufacturing
Sometimes when you want something done right, you have to do it yourself. We design and construct everything we offer – 100% in-house
6
Products in Pipeline
129
issued & Pending Patents
5
Products On Market


Our Team
Joseph C. McGinley, MD, PhD
Ben Warren
Richard C. McGinity, DBA
Diane McGinley, MS
Adam Johnson
Frequently Asked Questions
McGinley Orthopedics engineers “must-buy” surgical tools to eliminate the manual process and replace them with state of the art technology. Our two primary products are the IntelliSense® orthopedic drill and the Lever Action Plate System®. Each of these FDA-cleared products are currently in-market and represent the first-to-market product in an expansive future product line.
McGinley Engineered Solutions, a fully owned subsidiary of McGinley Orthopaedic Innovations, Inc., has 71 patent families including 104 issued patents and 22 pending patent applications.
MOI’s intellectual property is among its most valuable assets, consisting of:
- core technology/proprietary know-how/trade secrets
- issued patents (IP)
- pending patents
The Company will grow its IP as it conceives, designs, and prototypes new products. Potential strategic partners and acquirers see value in MO’s intellectual property assets and are seeking to acquire them.
McGinley Orthopedics is located in Casper, Wyoming. We have a full service in-house engineering team.
Yes. McGinley Manufacturing is a fully owned subsidiary of McGinley Orthopedics. MM is located in Glenrock, WY and is ISO 13485 and AS9100 certified. This company allows MO to control its own supply chain and ensure quality.
Acquisition of MM has also given MO several strategic supply chain advantages, including, scheduling priorities and lead times for the specialized medical parts, rapid prototyping capability for MO product engineers, eliminating substantial third party overhead and profit margins, cost reduction in the production of MO’s orthopedic surgery consumables (e.g. drill bits, saw blades, bone plates, pins). MM serves as an alternate source of revenue for MO by supplying high quality, precision parts to the mining, oil and gas, and manufacturing industries in addition to the specialized medical manufacturing parts. MM also offers “Concept to Production” services where the MO’s team of engineers work directly with customers to help in concept development, design, prototyping, and production.
Yes. McGinley Engineered Solutions is a fully owned subsidiary of McGinley Orthopedics. This company holds the company’s intellectual property.
Currently in the pipeline with expected release in 2023, the IntelliSense PinPilot revolutionizes pin/wire drivers. The IntelliSense PinPilot utilizes technological advances for pin and K-wire placement. With IntelliSense Drill Technology(R), this device can auto-stop after breeching a predetermined number of cortices, subchondrally, or endosteally while simultaneously providing depth measurement and cortical edge detection throughout the drilling process.
McGinley Orthopedics also offers accessories to expand the IntelliSense Drill’s use in the operating room. The following products from McGinley Orthopedics are designed to allow competitors’ accessories to be used with the IntelliSense Drill eliminating the need for other drills in the OR. They include a keyed chuck, keyless chuck, standard AO chuck and a universal pin driver. While these chucks allow the IntelliSense Drill to be used with a wide range of competitors’ products, the benefits of the IntelliSense Drill Technology(R) will not function including the auto-stop technology and depth measurement.
Hospitals rigorously schedule operating rooms to maximize utilization of these scarce resources. Two well-respected hospital studies estimate typical Level 1 Trauma Center OR costs in the range of $20.93 to $97.00 per minute, an average of $62.00, not including provider fees and anesthesia.19 The time savings from the automatic measuring features of IntelliSense® can add one procedure per day of OR utilization. An average trauma case uses 10 fixation screws. Surgeons spend one minute (or more) per screw to stop, measure and then re-drill each hole (10 mins x $62/min = $620). The IntelliSense® Drill eliminates the need to utilize a depth gauge as the measurement is given in real-time while drilling.
Fluoroscopy is used to confirm screw measurements. For each image, the team must move the fluoroscope to the patient, position the machine arm over the incision, and move team members away from the radiation. Then they take the image, move the equipment back out of the way, and finally reposition the team and resume drilling. In a trauma setting, this takes approximately four to eight minutes per case; in a spine procedure, 25-40 minutes. With IntelliSense®, surgeons can feel confident about screw placement and this process can be reduced or eliminated altogether. This can limit exposure to O. R. staff and patients.
Yes. Published literature indicates a screw error rate requiring removal and replacement of between 20% and 24.7%21 that is directly attributable to measurement error.22 Based on a minimum of six screws used per orthopedic surgery, we estimate 1 to 2 screw(s) requiring replacement per case. Fracture fixation screws cost approximately $35.00 to $150.00; uni-axial pedicle screws for certain spine surgeries cost up to $1,000 and multiaxial pedicle screws may cost up to $1,500. When surgeons recognize an incorrect screw length during surgery and replace it with one of proper length, hospitals often pay for the wasted, expensive and specialized screws at an estimated average cost of $120 per procedure.
IN MAKING AN INVESTMENT DECISION, INVESTORS MUST RELY ON THEIR OWN EXAMINATION OF THE ISSUER AND THE TERMS OF THE OFFERING, INCLUDING THE MERITS AND RISKS INVOLVED. REGULATION A OFFERINGS ARE SPECULATIVE, ILLIQUID, AND INVOLVE A HIGH DEGREE OF RISK, INCLUDING THE POSSIBLE LOSS OF YOUR ENTIRE INVESTMENT.
The SEC staff reviews certain forms and filings for compliance with disclosure obligations, the SEC does not evaluate the merits of any offering, nor does it determine if any securities offered are “good” investments.
This profile may contain forward-looking statements and information relating to, among other things, the company, its business plan and strategy, and its markets or industry. These statements reflect management’s current views regarding future events based on available information and are subject to risks and uncertainties that could cause the company’s actual results to differ materially. Investors are cautioned not to place undue reliance on these forward-looking statements as they are meant for illustrative purposes, and they do not represent guarantees of future results, levels of activity, performance, or achievements, all of which cannot be made. Moreover, although management believes that the expectations reflected in the forward-looking statements are reasonable, neither McGinley Orthopaedic Innovations nor anyone acting on its behalf can give any assurance that such expectations will prove to have been correct, nor do they have a duty to update any such statements to conform them to actual results.
By accessing this site and any pages on this site, you agree to be bound by our Terms of Use and Privacy Policy, as may be amended from time to time without notice or liability.


